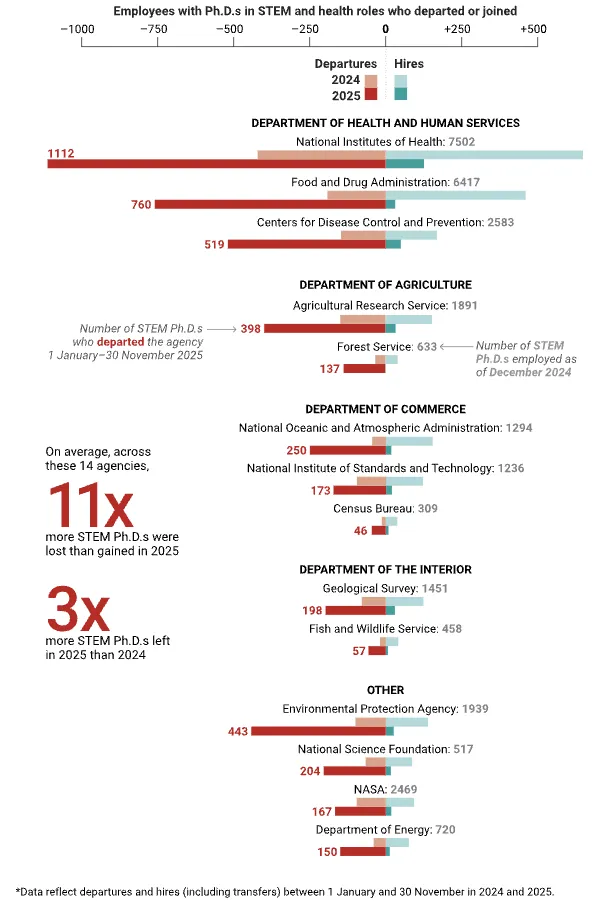
Intro. [Recording date: September 5, 2023.]
Russ Roberts: Today is September 5th, 2023, and my guest is Dr. Peter Attia. His podcast is The Drive and he’s the author, with Bill Gifford, of Outlive: The Science and Art of Longevity, which is our topic for today.
Peter, welcome to EconTalk.
Peter Attia: Thanks for having me, Russ.
Russ Roberts: Now, the book is a combination of advice for the reader: how to live in a different way. It also is a critique of advice for the field of medicine. And I’m sure we’ll bounce back and forth between those.
I want to start with an early part of the book, which is a dream you had, maybe more of a nightmare, that involved eggs. Tell us what that was about.
Peter Attia: Yeah. So in this dream, I was sort of tasked with catching eggs before they hit the ground. And so, there’s a tall building–not that tall, maybe two or three stories tall–and there’s a guy on the top, and he’s tossing eggs out of a basket. And I have a padded basket beneath him on the street level, and I’m running around trying to catch them all. And, truthfully, I’m not doing that well. I’m catching some, but many of them I miss and they splat all over the pavement.
And, of course, this dream, I realized many years later–I mean, probably a decade later–probably spoke to how I felt in my medical training. I had this dream during Residency, and it probably spoke to the feeling that we were doing so much at the last minute to try to save lives. And, of course, we could. We had some remarkably heroic things that we could do. But, ultimately, they were typically futile, and they merely extended lifespan slightly without really meaningfully impacting healthspan.
And, of course, in the dream, I come to realize that the only way to stop this is to go to the top of the building and take the basket of eggs from the guy who is tossing them.
Russ Roberts: Explain the difference between lifespan and healthspan.
Peter Attia: So, lifespan, I think, is the easier of these two to understand. It’s certainly the easier to measure, because it is binary. It’s discrete. And it’s the period of life in which you are alive: you’re respiring.
And so, we measure lifespan. And most of the data that is collected with respect to health is lifespan-based.
And, you think about it this way, Russ: Everybody says, ‘Well, median lifespan or maximal lifespan has extended by such and such amount over the past hundred years.’ And, if we look at those metrics, we can clearly see successes. So, if you look 150 years ago versus today, we have indeed doubled the expected lifespan of a person living in the developed world from approximately 40 to 80. We can talk a little bit about why that’s happened.
Healthspan, however, is the analog measure. It really speaks to quality of life.
Now, I tend to have a more nuanced definition to this than the medical establishment would today.
The medical definition of healthspan is the period of time in which you are free from disability and disease. I don’t find that to be a particularly helpful definition. It, maybe, lends itself to slightly better measurement than my definition, but I don’t think it truly captures what people care about.
I think what healthspan really comes down to is the quality of your physical life. So, your ability to move around, do things, be free of pain. It’s the difference between the 80-year-old and the 30-year-old from a physical activity-level standpoint. There’s a cognitive component to healthspan, and this is absent disease, so we’re not talking about dementia, but we’re still talking about executive power, processing speed, memory, those things.
And then there’s an emotional component. And this is the more interesting one, in a sense, because it’s the only one that doesn’t decline with age. It doesn’t necessarily have to decline.
I think, unless you’re living in a sci-fi land, we have to accept that our physical and cognitive healthspan is inevitably going to decline as we age. This does not have to be true with our emotional health, which includes everything from our sense of purpose, the quality of our relationships with others, with ourselves, our happiness, joy, spontaneity–all these things.
And so, to me, those are a basket of things that encompass healthspan. And, I will say this, Russ: when patients come to me, virtually without exception–because I always ask my patients on the first day, ‘What is your goal for the last decade of your life, this marginal decade?’–they virtually never talk about lifespan. They only talk about healthspan.
And, I think therein lies one of the biggest problems with our healthcare system today is the relentless focus on lifespan. By the way, we’re not even doing that well, so we can talk about that. But, to the exclusion of healthspan.
Russ Roberts: Yeah. I love–the fundamental idea behind this book, is that of course, we don’t want to just live long. We want to live in a meaningful way, as you say. We want to live in a way that brings delight and that is–maybe diminished relative to our youth, but it still at least reminds us of some of the aspects of our youth in terms of what we care about doing and how we spend our time. And, so much of our focus is on saving lives and not on the quality of life. And, you make the distinction between Medicine 2.0 and Medicine 3.0, and you can also start with Medicine 1.0. So, give us those three eras.
Peter Attia: So, Medicine 1.0 represents the longest in terms of time because it’s basically medicine for all of human history until about the transition that occurred from, say, Francis Bacon to germ theory. That’s really the transition to Medicine 2.0.
So, in Medicine 1.0, we didn’t understand the natural universe. Right? The scientific method had not been created; and while we can look back at what people thought at the time and laugh, I think we have to be a little bit kinder and realize that you can’t use a tool that doesn’t exist. And so, if a person was ill, you had to assume this was the gods or there were bad humors in the air that were causing this.
All of this changed, of course, in the 17th century when Francis Bacon began the work that I think led to the elucidation of the scientific method. There were also some really big technological breakthroughs. I’m sure you’ve read Sidd Mukherjee’s book The Cell, and I think he does perhaps the best job of anyone in kind of explaining what really took place in this cellular revolution, where we finally began to understand that there were microscopic organisms that played a role in our illness. And so, the development of the light microscope and ultimately the discovery of antimicrobial agents completely changed the face of medicine.
And that is where I really think Medicine 2.0 took hold, in the late 19th century. And by the way, that is really the thing that is accounting for the doubling of human lifespan in the past 120 to 140 years.
There is, of course, another very important tool in Medicine 2.0 that was not introduced until about the 1940s, and that was the statistical and conceptual tool of the Randomized Control Trial [RCT]. This is what really allowed Medicine 2.0 to take off and to continue to make advances in other areas such as oncology. And in particular, I would say the poster child for Medicine 2.0, beyond the obvious–which is communicable and infectious diseases–is in cardiovascular disease.
So, we have Medicine 2.0, which is currently the system that we’re in. It does very well, again, for infectious and communicable diseases, and it also does very well for acute injuries. So, our ability to actually deal with a heart attack when it’s happening today is remarkable compared to 50 years ago. Our ability to take care of trauma patients, to take care of patients in sepsis, it’s unparalleled.
However, what this has all shown us is that Medicine 2.0 has an enormous blind spot, and that is to the treatment of chronic diseases. We’ve made virtually no progress in the management of chronic diseases, and that’s why today we see that the majority of people living in the developed world are going to die, indeed, from chronic diseases.
Russ Roberts: Yeah. When we think about that expansion, that doubling of lifespan, it’s overwhelmingly two factors: the incredible improvement in childbirth–you talk about Semmelweis. We’ve talked about Semmelweis many times in this program–a tragic figure, incredibly poignant–who understood that it was washing your hands after you went to the morgue; and no one believed him. And the tragedy for me has always been he was careless about his study because he was so confident he was right, and so it made it easier for people to dismiss it; along with his personality; and of course, that led to the deaths of thousands of women prematurely. Such a sad story.
But, we have the improvement in childbirth and in childhood death–death before the age of five. So, once you reached a certain age, six, ten, fifteen, whatever it was in the data–I’m not sure, but five is a good round starting point–the improvement isn’t anything close to a doubling. Once in 1900 you reached age of five, many people lived to be 70 and 80 years old. It wasn’t everybody died at 40, even though that was the average. That’s because you had a lot of zeros and point fours for the people who died as infants.
The second improvement, though, that–I knew that before, but what I learned from your book, which I didn’t realize or appreciate–is that most of the improvement since then is in fighting sepsis, infection and the use of antibiotics. Which is a miracle, an extraordinary revolution in human creativity. But, the other things, very, very, very, very slow progress or none, and it’s easy to forget that. We hear lots of statistics about improved mortality from certain conditions. They’re somewhat misleading in that sometimes it’s due to earlier detection rather than actual improvement in outcome and mortality outcome.
And then, there’s the question that you focus on in the book, which I find really a totally different perspective, which is that our goal isn’t to keep people alive. It’s a cliché, but it’s so easy to forget it. It’s to have a good life. And, it’s not just, ‘Oh, take risks and enjoy things, because of course you’re not going to live forever.’ It’s that we may have the potential, and medicine could focus instead on making sure that you maintain function further and further into your lifespan. It’s a beautiful idea.
Peter Attia: Yeah, and I think I want to reiterate a point I made very quickly, but I think it’s the jugular issue here, which is the old cliché, what gets measured gets managed, could not be more true. And, in Medicine 2.0, understandably, the benchmark is lifespan, and therefore, everything works towards the management of lifespan. If we instead chose to focus more on healthspan, I believe we could be more effective. But, it’s also understandable why we’re not focusing on healthspan. It is much harder to measure, and therefore, I think it’s easy to understand why we as doctors will instead focus on the metric that we can measure.
Russ Roberts: And we’ve had recent episodes with a number of folks we’ll link to, pointing out that a lot of end-of-life care, it isn’t just that it extends your life a small amount: it extends it in unpleasant ways often that take out the opportunity to do the things we love. And, maybe we’ll talk more about that later. But, what are the Four Horsemen of the Apocalypse? What are the four things that kill us slowly?
Peter Attia: So, if you’re a non-smoker–and that’s an important distinction because if you’re a smoker, there’s a fifth really big bucket here, which is chronic lung disease. But, if you take out lung disease, aside from lung cancer, the Four Horsemen, in order, are: cardiovascular disease or the diseases of atherosclerosis–so that includes cerebrovascular disease, strokes, heart attacks, heart failure, all of those things.
That’s hands-down Number One, Russ, and it’s funny how little attention it gets. At least in America, I think cancer gets a lot more attention, perhaps because it’s scarier. But, the truth of it is, heart disease has always been Number One. And, by the way, globally, that gap is even larger. So, globally, that gap is about 19 million versus 13 million. That’s deaths per year.
Disease Number Two is cancer; and again, we have to be careful when we talk about cancer. It’s not a disease, right? Cancer is a very, very heterogeneous set of diseases that only have two things in common–which we can talk about–but aside from that, completely different process. Right? So, breast cancer and colon cancer are so different that to compare them–to say that they’re one disease called cancer–is a little bit misleading.
The Third Pillar, or the Third Horseman, would be all of the neurodegenerative diseases and all of the diseases of dementia. This is a bit of a two-bucket, if you will, because not all forms of dementia are neurodegenerative. For example, vascular dementia, which is a significant driver of dementia, is not neurodegenerative the way Alzheimer’s is; and of course, Parkinson’s disease is not typically a dementing disease in the way that Lewy body dementia or Alzheimer’s is. But collectively, taken together, this would be the Third Horseman.
The Fourth and final Horseman is one that doesn’t really directly kill an enormous number of people, but indirectly, I would argue, is the gasoline that is poured on the fire of all of them, and it’s the cluster of metabolic diseases, or maybe a better way to think about it is the continuum of metabolic diseases that ranges from insulin resistance all the way to Type 2 diabetes. Now, Type 2 diabetes is indeed an epidemic in the United States. I apologize: I can’t quote worldwide statistics. But, if you just go back in time 50 years to when I was born, the prevalence of this condition was less than 1% in the United States. Today it’s over 10%.
So, when you have a log-fold increase in a disease in a decade–and that is not a diagnostic difference; I mean, it’s crystal clear that using the same diagnostic criteria, we have this log-fold increase–it’s clear that there’s a problem. And that problem, while it does kill people directly–people do die directly from Type 2 diabetes–far more likely they’re dying as a result of it’s doubling of risk of these other Horsemen.
Russ Roberts: Yeah. That’s a fascinating point.
Russ Roberts: I have to say, when I started your book–and it’s very entertaining. It’s very well-written. Besides the medical insights and advice you give, there’s a great deal of you in the book, which is riveting. I have to say, when I started it, I had a lot of skepticism, because if you asked me before I read your book, ‘These four things, we want to avoid them and we want to do better,’ I would say, ‘You know, other than maybe obesity,’–which we’re going to talk a lot about–‘and diet and nutrition, these things are overwhelmingly driven by genetics. And, right now I can’t change my genetics, at least in our current medical knowledge, scientific knowledge, but I can be kind of cavalier about these risks, because it’s already written into my DNA [Deoxyribonucleic acid].
And, in fact, ironically, you start with an analysis–after the introductory parts of the book–you start with analysis of people who live very, very long. They live over a hundred. Centenarians. And, they smoke. They drink. There doesn’t seem to be anything they have in common behaviorally. So it kind of reinforces my bias.
And yet, you come up with a very clever insight about those folks and use that as an example of why my initial skepticism might be misplaced. So, while it’s true that genetics have a lot to do with our risk of heart attack, cancer, Alzheimer’s, etc., it’s not the whole story.
Peter Attia: Yeah. So, this is an area where, Russ, I had no idea what the answer was going to be ten years ago. So, I didn’t start writing this book till seven years ago. But of course, the topic was my life’s work.
And so, 10 years ago, I was really digging into this particular question around centenarians. So, these are the people who–as you point out–live to be a hundred and beyond. And there’s something very special about them. And understandably, there are no shortage of studies being done on them. But the two people who have done the lion’s share of this work are Tom Perls at Boston University and Nir Barzilai at Albert Einstein.
And I’ll never forget the day when I finally–you know when Neo in the Matrix finally sees the connection and everything? I’ll never forget my Neo-in-the-Matrix moment, which was literally, I don’t know, circa 2011. And, up until that point, there was a very important question I wanted to understand, which was: did centenarians somehow have immunity from chronic disease, and therefore, it was just a matter of time before something else killed them that was not part of the chronic-disease playbook? Or did they just delay the onset?
And, it became really clear, again, just through putting all this work together 10, 12 years ago, that the answer was: No, they’re actually not immune to chronic disease. They just have a phase shift in time of 20 to 30 years, but then they basically die in the exact same distribution as the rest of us, with a slight difference. They get slightly more cardiovascular disease and slightly less cancer. But–and I talk about all this in the book–when they get their first heart attack, it’s about 20 to 25 years later; but then they go down the exact same path to demise of cardiovascular disease. When they get cancer, they just get diagnosed 20 to 25 years later, but then their path towards death is the exact same.
So, the insight here–the first insight–was centenarians’ gift is indeed delaying the onset of chronic disease. Not avoiding it indefinitely, simply delaying it.
The second insight, and this is the second part that the chapter focuses on, is–and you led with this–it’s not due to any behavior. It truly is a genetic lottery. Contrary to popular belief, however, it’s very polygenic. It is not like there is a longevity gene. Now, there are some that show up more frequently, and I talk about the top four or five in that chapter, but I talk about them not because you and I can do anything about them.
You and I are not going to go change our APOE [apolipoprotein E], our CETP [cholesteryl ester transfer protein] gene, our FOXO [Forkhead Box O] gene, or any of those genes. However, what we can do is copy the phenotype. We can’t change the genotype. We can’t change the actual gene to match theirs, but we can learn what that gene is doing.
So for example, a [?]–a mutation in a gene or a snip in a gene, a difference in a gene that changes or lowers the burden of their lipoproteins, the causative driver of atherosclerosis, is going to lead to a delay in the onset of atherosclerosis. That’s something we can mimic.
So, the other thing that came out of this study for me was the understanding of where and when genetics play a role in lifespan. And it turns out that up until about the 8th decade of life, genes play virtually no role. You can never say no role, because there are examples I could give where genes do indeed–are almost deterministic in lifespan. But those are absolutely marginal cases. For the most part, into your 8th decade, genes don’t play any impact on lifespan. However, as you get into the 9th, 10th, and 11th decade, genes start to matter.
All of this comes back to, though: So what? Why does this matter? Well, I’m not here to suggest you or I–I don’t know your family history, Russ, but let’s just assume you don’t have centenarian parents or grandparents or siblings. Well, you wouldn’t have siblings, but parents or grandparents, aunts and uncles. And, I certainly don’t. I have very little belief that anything I’m going to do is going to get me to live to be 100, but that doesn’t mean I can’t live, say, five, six, seven years longer than my, quote-unquote, “genetic predisposition.” And again, and more importantly, it’s: What can I do for my healthspan? What can I do such that at 80, instead of looking and functioning like the average 80-year-old, I could look and function like the average 65-year-old? That’s the really important thing to consider.
Russ Roberts: And, that’s very inspiring and very moving.
Russ Roberts: I want to raise a question you don’t talk about in the book. It feels to me that–my mom is 90. My dad died at 89, and he was a smoker, and he had about five horrible cancers over the last 15 years he was alive. They didn’t eliminate his ability to play tennis, ride his bicycle, spend time with his grandchildren, but it took a lot of the fun out of it. He hated the phrase ‘he battled cancer,’ like it was a military campaign. In his view, it was just you were stuck with it, and you did what you could. And, he was lucky in a sense that he overcame them for a while, but while he was overcoming them, it was really unpleasant. So, that theme of your book really resonates–really resonated with me.
But, let’s take my mom. My mom is 90. She lives on her own. Until about a month ago, she drove–until she knocked her side mirror off. Her car misbehaved. It accelerated suddenly when she put her foot on the brake; and she decided it was a design malfunction. Her children thought otherwise.
But, she’s lucid. She takes walks every day. She lives a very good life doing the things that she still enjoys. At 90. My wife’s mother just turned 80, and she’s also very active. It feels to me that 90 is the new 80, and 80 is the new 70. Is that a misperception on my part, because when I was younger, old people just looked really old, and a 70-year-old seemed decrepit and hopelessly having a hard life? Or have we made some improvement in healthspan already?
Peter Attia: No. I wish you were right, Russ. I think that’s a sampling bias. I think you have a couple of really great examples.
Russ Roberts: Small samples.
Peter Attia: Yeah. I think you have a couple of wonderful examples in front of you; but sadly, I don’t think that’s the case. I think we’ve done the opposite, actually.
So, there’s two things going on, actually. Let’s go back to the example that you gave. Believe it or not, if in the 1940s someone managed to escape childhood mortality, managed to escape all of the infections that probably would have killed them along the way–managed to not get a heart attack, i.e., managed not to die by the time they were 85–they were on balance, far more robust than the 85-year-old today, in large part because of their lifestyle. Right? They were a far more active person, they were sleeping better, they were probably under far less stress. There’s a lot of things that they had going for them. Whereas I think today, yes, we do have more people living to that age, but they’re living at probably a much lower quality of life. So, I think the two women in your life are excellent examples of what we would aspire to.
Russ Roberts: Yeah. And, I’m, of course, well aware that they just could be lucky, and I may not be, or may be, no way of knowing in advance.
Russ Roberts: Now, you talk about five tactics that we’re going to talk about: Exercise, nutritional biochemistry, sleep, emotional health, exogenous molecules. And, you have interesting things to say about all of them, and we’ll get to that. But, before we do, I want to talk about screening, because you make a case for–one of the Four Horsemen is cancer, obviously. We’ve made tragically little progress in, quote, “curing” it, or with a couple exceptions, we’ve made very little progress in reducing mortality dramatically.
Vinay Prasad, recently on the program, very skeptical of all-mortality impact for screening for breast cancer and colon cancer. We can talk about many other things, but he’s certainly skeptical of those. I think you’re less skeptical on the colon cancer front. You have a different interpretation of the NordICC [Nordic-European Initiative on Colorectal Cancer] trials. I’m going to give you a chance to talk about that. I have a thought in response. But, talk about your view of screening and in fighting cancer, and then we’ll turn to this question of tactics for better health span.
Peter Attia: Yeah. I think my thinking on screening has really changed so much over the past decade. I trained in cancer–so I did my fellowship at the National Cancer Institute in immunotherapy, and got to see firsthand what I think and what I believe is the most promising therapy we have for cancer, going forward.
So, everything you said about the lack of progress in cancer so far is true, and I’ll put some statistics to that in a moment. But, when I think about the next decade, I’m actually much more optimistic; and we can talk about why. But, let’s talk about some facts.
So, 50 years ago–a little over 50 years ago–Richard Nixon declared a war on cancer. The goal, if you recall–this is coming on the heels of the success of the space project where Kennedy declares, ‘We’re going to go to the moon,’ in 1961; and in 1969 we’re there. And, let’s be clear: it was a very reasonable, although naïve, thing to assume that we could do the same thing with cancer.
In the early 1970s, if you had metastatic, solid-organ cancer–so epithelial cancers mean cancers of breast, prostate, colon, pancreas, lung, brain, all those things essentially–if you had metastatic, solid-organ tumor, the probability you would be alive in 10 years, was zero. That’s the metrics that matters. Not median survival. Median survival might have been 12 months. Today it’s longer. But, overall survival, zero.
What is it today? Today it’s slightly better than zero, but in solid-organ tumor, there’s only two solid organ cancers that are being cured today: testicular cancer–which was not cured back then–and a very rare type of stomach cancer called GI stromal tumors [gastrointestinal stromal tumors]. This is not the stomach cancer that killed most people. This is a rare cancer because it happens to arise from one mutation for which there’s a beautiful, targeted drug.
So, we’ll make the math easy and say 50 years ago, a hundred percent of people with metastatic cancer died in 10 years, or [0%?–Econlib Ed.] were still alive in 10 years, and today 1% of those people might be alive. So, that’s really low progress. Now, median survival has increased significantly. It’s probably more than doubled, and some non-solid organ tumors have become cured. So, the success against leukemias and lymphomas has also been remarkable.
Again, I think for the space of time, we don’t need to go into all of those, but I just want to make sure people understand that: look, overall survival for cancer–overall survival–is probably up by 8-10% from 50 years ago.
But, why so little? Well, I would say that there are a couple of things going on, but one of them is–the obvious is–we don’t have great treatments. So, we don’t have great treatments once cancers reach a certain size and a certain mutagenic burden. The cancers seem very good at outmaneuvering therapies.
But, the other thing I would argue, is we’re intervening very late, because we intervene at a time when the cancer is quite visible. Now, if you consider, I don’t know, a lump in a breast that turns out to be cancerous, it’s already, even just at that one lump, well over a billion cells. Now, the question is: What’s the probability in a billion cells that you are going to have heterogeneous pools of cells that are not going to respond to the same therapy? It’s actually pretty high. It’s much higher when that number is a hundred billion cells.
So, enter the discussion of screening, which is something I used to really be a stronger skeptic of. I really used to think that screening wasn’t doing much. In fact, I even worked on a lengthy project on this when I was in medical school, and I used to think that most of the effective screening was lead-time bias. So, you kind of alluded to this actually a few minutes ago, Russ, when you said if you catch a cancer two years earlier, it could look like you’re living two years longer, when in fact you’re not.
I think–a couple of things now, I feel different about this. So, the first is the following. If you look at colon cancer and breast cancer, which are two easy examples to look at–and I write about this in the book–we compare the success of adjuvant treatment versus metastatic treatment–so I’ll define those terms in a second–you see an important pattern. Adjuvant treatment is the treatment you give a patient after you have removed all visible cancer. So, this is what’s done in Stage II, Stage III colon cancer.
So, a Stage III colon cancer–let’s use that as an example. A person has a colonoscopy and you see a tumor; they sample it, they biopsy it. Yep, they know it’s cancer. That person goes to the operating room; they have a section of their colon removed and a section of the adjacent tissue, and you actually see that the cancer even went to the lymph nodes. So, that’s a pretty aggressive cancer. But, during the surgical exploration, the surgeon looks; there’s no visible tumor in the liver. You can see that. You do a scan of that person, there’s no visible tumor anywhere in the body. So, the term in oncology is: that person is NED–No Evidence of Disease. Do we just leave that person alone? No, we don’t. We give them a cocktail of chemotherapy. What’s the survival of that person five or 10 years later, call it 10 years later? It’s a little over 50%, meaning half those people are alive–a little over half those people are alive–but half those people had a recurrence, typically in the liver or in the lung, and they don’t survive.
Now, what about the patients who have metastatic cancer on that same day that these persons had their surgical resection? It turns out we give them the exact same chemotherapy. How many of those people are alive?
Russ Roberts: Explain what metastatic cancer is. In other words, the first one was no visible tumors, but the cells nearby have some–have cancer.
Peter Attia: That’s right. In the metastatic case, it’s visible to the naked eye. Either at the time that the surgeon removes the colon, he or she can actually see cancer in the liver, or even just doing a scan on the person you see that the cancer has spread to the liver, lungs, where else. We give those people the exact same therapy. Zero of those people are alive. So, what’s the difference–
Russ Roberts: Five to 10 years later, you said–
Peter Attia: That’s correct.
Russ Roberts: Right.
Peter Attia: That’s correct, yeah. What’s the difference? Why is it that the people who present with metastatic disease cannot survive for 10 years, where those that present with Stage III disease and you give them the exact same therapy, you could save half of those people permanently? That’s a cure, by the way.
I believe–and many others as well–believe that the difference is in tumor burden. The difference is that when you’re treating that patient with adjuvant therapy–i.e., when you don’t have visible tumor–you probably only have a few billion cells scattered throughout the body. When you’re treating that patient with abject metastatic disease, there are hundreds of billions of cells throughout the body. It’s a numbers game, and the numbers are not in your favor.
The same example, by the way, is true with breast cancer. So, if you go hormone receptor for hormone receptor and do the same exercise, when you treat women for adjuvant therapy versus treat women for metastatic therapy using similar regimens, you’re going to get similar outcomes [similar to those when treating colon cancer–Econlib Ed.].
All of this leads me to the inevitable conclusion that the earlier one can detect a cancer and treat it, the better your odds are. Does it mean that there’s a guarantee? Absolutely not. The biology of this is very complicated.
And by the way, I think if we want to talk about breast and colon, those are two great examples, because they have very different biology. So, colon cancer develops in a Halstedian manner. So, William Stewart Halsted, the modern architect of American surgery, proposed this idea–which is not true for all cancers, but is true for colon cancer–and that is the stepwise progression from adenoma to cancer to distant spread. So, every single colon cancer comes from a polyp, though most polyps do not become cancer.
This is not true in breast cancer, and this is why I believe breast cancer is a much more complicated cancer–right?–we don’t fully understand. Not every breast cancer comes from a ductal carcinoma in situ. So, there’s something going on in the biology of breast cancer that makes it more complicated.
But, the reason I do feel very strongly about colonoscopy, is it’s also one of the few cancers you can look directly at. You can’t look at breast cancer. You have to infer it from a mammogram, an ultrasound, an MRI [Magnetic Resonance Imaging]. Those are all flawed. They all have limitations in sensitivity and specificity, the way a colonoscopy does not.
So with colonoscopy, I think what we really need to be considering, is: At what frequency would you need to do this, given that you know every cancer came from an adenoma, to justify the risk? Because, we’ll have to talk about the three risks of colonoscopy. And it’s not a benign procedure–unlike a mammogram which has a trivial amount of radiation and is effectively a benign procedure.
So, I don’t want to suggest that this is an easy problem to solve, and that’s why I write about it at length. But it is–and again, I have yet to see evidence that suggests that treating advanced cancer is better than treating early cancer, or treating a hundred billion cells is better than treating a billion cells. Or as good as: let’s put it that way.
Russ Roberts: So, the question is–as we talked about in the episode with Vinay Prasad, the all-cause mortality–which I misspoke a minute ago–all-cause mortality for colonoscopy seems to be relatively unchanged by the procedure: either because it doesn’t matter–meaning it’s not that important to get it early. Probably not the reason. There are side effects that you alluded to, the three things. There’s perforation, there’s infection; I assume there are other things. And, while you’re skeptical, I know of some of those all-cause mortality results, they are discouraging. And attempts to–it does call into question the–it’s complicated, but–
Peter Attia: Well, let’s talk about the NordICC trial, because I think that’s the trial that would be looked at as the case against colonoscopy from an all-cause mortality standpoint. So, the NordICC trial, just for folks listening, is an enormous trial that was done in Europe, published recently. It was about a year ago; I think it was published last summer. And it looked at two groups of people. Half the group was randomized to usual care, which meant not doing anything. The other half was randomized to a recommended colonoscopy in a 10-year window of time.
Well, let’s pause for a moment and make sure people understand the difference between effectiveness trials and efficacy trials.
So, an efficacy trial says–let’s use a different example to make this easier. Let’s say, Russ, we wanted to study the effect of a certain diet on weight. So, we get our people, we randomize them, and we tell one group, ‘Look, you’re going to have to eat lettuce and carrots and lima beans and Fiber One cereal for the next five years. That’s all you’re allowed to eat.’ And the other group, ‘You can eat whatever you want.’ And, five years later we go and see how they’re doing.
Well, the efficacy of that is only determined if the people are able to adhere to the right diet. And, the notorious challenge of dietary studies, is: it’s almost impossible to do efficacy studies if the intervention is quite extreme.
For the most part, we give up on doing that, and we only focus on effectiveness studies, which is: how effective is this intervention in the real world, i.e., how effectively can people follow the advice that is given? So, again, when you tell people, ‘Go on a low fat diet, go on a Mediterranean diet,’ they’re going to adhere to it at some level, but it’s nowhere near the prescribed level.
So, this was an effectiveness trial of colonoscopy: We’re going to recommend that you guys get a colonoscopy once in 10 years. 40% of them did. A). I’m not convinced that every 10 years is nearly sufficient enough. There’s literature showing that people can develop colon cancer as little as one to two years after a completely normal colonoscopy. Again, we could argue maybe in those cases the adenoma was missed a year earlier. It’s hard to say. But what I would say is 10 years is far too infrequent.
And furthermore, we didn’t have very good compliance, despite a statistical analysis that suggested that didn’t matter. I don’t buy it because I also don’t think that the 10 years was sufficient.
So, I think that what we really ought to be doing is asking: What is the probability of one of those three risks?
So: What are the three risks of colonoscopy? There’s the risk of the bowel prep–which for people like you and I is not an issue, Russ, but it would be for your mom. And by the way, I’d have reservations about your mom doing a colonoscopy because of the preparation. She could get so dehydrated that she could fall and knock her head, right? The bowel prep is a big deal. But that’s risk number one.
Risk number two is the risk of the sedation. It’s a low risk, but it’s not zero.
And then risk number three is the procedural risk of bleeding and perforation. And, I always advise people, when you’re getting a colonoscopy, you must ask the endoscopist what his or her risk is of perforation.
Russ Roberts: I’m sorry for laughing, I apologize, because I think I recently, in the Prasad episode–I don’t know if it was in that episode or not–but a friend of mine was recently encouraged to do a particular procedure, and I was worried about it, about a bad outcome, and I said, ‘You should ask about it.’ And the guy said, ‘Well, the odds of it are only,’ blah, blah, blah. And I said, ‘You don’t want the national odds. You want his or her odds’–
Russ Roberts: ‘the person doing it.’ And, I assume they’re not so eager to tell you, sometimes.
Peter Attia: Yeah, but, you know, look: we were encouraged in my surgical training to be very open about that. Even for the simplest procedure, like putting a central line into somebody, I could quote the national average, but I could also tell you that in the last 300 of these, I punctured one lung. In the last hundred times you take out somebody’s gallbladder, you accidentally nick the common bile duct–whatever the number might be. So, I don’t want to downplay any of those risks, and they must be taken seriously.
But, what we really need to do is ask the question: What is the cost of those versus the cost of missing a colon cancer?
So, here’s the thought experiment. So, as an economist, you’ll appreciate this, because the only way you can do this thought experiment is to throw money out the door for a minute. So, we’re not going to talk about the economics of this.
Here’s the thought experiment. If you did a colonoscopy every three months on a person, could they ever die of colon cancer? If you did a proper bowel prep and did a colonoscopy, the answer is No. Right? There’s no chance that in three months an adenoma could develop and become cancerous in three months.
So, the question then is: Okay, so you take the 53,000 people a year in the United States who die of colon cancer, you took that number down to zero. You have to, because it’s a Halstedian cancer. Now the question is: How many more people did you kill from the bowel prep, the sedation, and the perforation?
By the way, I did this calculation on the back of my napkin one day: I got about 5,000. You would kill about 5,000 people doing procedures.
Should we make that change? I’m not suggesting that at all. I’m suggesting that’s an extreme way to think about it, right? You take–
Russ Roberts: Yeah, that’s a nice–
Russ Roberts: There’s also the stress, which is, I’m not sure how that factors in, how important it is, [inaudible 00:43:44]–
Peter Attia: And that’s a bigger issue with some of the other cancers, Russ, I think. I think that’s less of an issue with the colon cancer, because you get the answer right away. But, I think your point about stress is significant when it comes to the more vague imaging modalities. I’ll warn all of our patients–
Russ Roberts: You get false positives–
Russ Roberts: You get false positives. I’m thinking more of the stress of the procedure. A lot of people, because of our cultural taboos, the idea of a colonoscopy is so unpleasant that some of the 60% who didn’t get that, even though it was recommended in that NordICC trial, just didn’t want to think about it. And if you’d forced them to have it, or in some measure incentivize them, it would come with an additional burden. But, it’s probably pretty small. So, I think Vinay disagrees with you, but I’m not sure why. He has reported on–
Peter Attia: Well, I’ll tell you why, I think, having not spoken about this with Vinay, though I’d be happy to. I think Vinay and Peter come at this through totally different lenses, and neither is right and neither is wrong. Right? Vinay is coming at this through the lens of policy. He’s coming at this through the lens of: What is the recommendation to all?
I’m coming at this through the lens of the individual. I’m giving you, as a person, a way to think about this for your decision around colon cancer. I’m going to get a colonoscopy every three to four years, because I am comfortable with the additional risk that I’m taking of the procedure. By the way, the additional cost I’m bearing–because my insurance will not pay for it more than every five years, so I’m personally on the hook; and it’s not cheap; I mean, I just paid for my last colonoscopy–I think it was about $2,000. So, that’s a big sum of money. But, at the individual level, I can do the math and make that determination.
I think Vinay is saying we’re trying to make recommendations to millions of people here. It can’t be that nuanced. It has to be based on effectiveness trials, not efficacy.
Russ Roberts: He claims they’re the same: that after a statistical analysis, that 40% or 60% difference wasn’t important. But, that’s a separate statistical question. Reasonable economists and data analysts can disagree on it, I’m sure.
But, I think it’s important for our listeners who may have, after the previous episode said, ‘Well, I’ll never do that again,’ should think twice. And I appreciate your view on this.
Russ Roberts: I want to turn to nutrition, which is one of the tactics you talk about. First, let’s get it off the table: You’re not a big fan of epidemiology; and you and I share a deep, I think, brotherhood there.
Peter Attia: Yeah. I used to be far more outspoken about it, although I do devote some real estate in this book to talking about how we need to be very skeptical of nutritional epidemiology. I think epidemiology has had some enormous wins, and I always try to point those out–to sort of say: Look, the field in and of itself has done some good. I mean, we can point to cholera and other things like that. And certainly smoking, by the way: We were never going to do the definitive randomized control trial to demonstrate the harm of tobacco smoke.
But, I also have to explain the Bradford Hill criteria to make sense of when can you increase your confidence in the causal nature of an observation? And, I think it is very safe to say, Russ, that the ability to infer cause from nutritional epidemiology is somewhere between zero and epsilon, where epsilon is a very, very, very small number.
Russ Roberts: Yeah. So, but: that does not mean we have nothing to learn about nutrition. And I want to give you my takeaways from your book and let you elaborate on them.
So, I would say a huge theme of the tactical part of your book–what we would call advice–and it’s wonderful, because you don’t say, ‘Go Paleo. Lift weights. Do this with kettlebells.’ You are a wise man. You understand that everybody is different, and a lot of those things, those disputes about various diets and exercise regimes are, as you say in the book, religious, rather than intellectual. And, it’s a wonderful insight, very important.
But, you spend a lot of time on exercise and nutrition without giving explicit–without joining a church, which I really appreciated–but, they’re quite complicated, a lot of your suggestions. And I want to start with the simplest recommendation I would take away from your nutrition, and then see if you can make me go a little further.
So, kind of simple, actually: Don’t be obese, take in fewer calories, stay away from sugar and junk food, and you’ve gone a long way toward what nutritionally you can do. There’s more to say, and I want to let you say it, but I think that’s an enormously big step for most people. And I would say, as a person on a diet right now, I’ve been deeply inspired by the section of your book on calorie reduction, to stick with it. So, I’m guardedly optimistic: you’ve had a powerful impact on my beliefs. It may be a placebo effect, but that works, too. So, I’m okay with it.
Peter Attia: Well, I’m glad to hear that, Russ. I think the easiest way to talk about nutrition is to maybe do it through the clinical lens in which I will look at a patient. So, every time I interact with a patient, I’m asking myself three questions. I mean, I’m asking myself hundreds of questions, but these are the three questions that factor into the thinking around a nutritional strategy. The first question is: ‘Is this person over-nourished, or adequately nourished?’ That’s a way of saying, ‘Are they storing excess energy or not.
Let’s understand something here, which is up until about 150 years ago, i.e., from 250,000 years ago until 150 years ago, the ability to store excess energy was very good. Right? This was our Darwinian superpower. This is what allowed us to leap forward. Our brains, which weigh 2% of our body weight, consume 20 to 25% of our metabolic demand. There is no way our species could have evolved to where it did, did we not have the capacity to store enormous amounts of energy. So, this was a very good thing–until very recently when we entered such an energy dense environment.
So, Question One is: Are you storing excess energy or not?
Question Two is: Are you adequately muscled or not?
And, Question Three, which is often related to the first two: Is are you metabolically healthy or not?
Now, the good news is all three of those questions have objective answers. There’s nothing subjective about this.
Now, you can’t measure them on a bathroom scale. You do need more complicated testing called DEXA [Dual X-ray Absorptiometry] testing, but that’s readily available. It’s about a $100 test. Everybody can get a DEXA scan, and understand the extent to which they’re over-nourished/under-nourished, adequately muscled/under-muscled. And I explain all the parameters for these things, and if they’re metabolically healthy or not–which can be done with blood testing. [More to come, 51:36]