Michael Fischer
Incannex Healthcare Inc. (NASDAQ:IXHL) is a developer of medicinal cannabinoid and psychedelic pharmaceutical product candidates. The company was incorporated in 2001 and is located in Australia. Its strategy is to combine cannabinoid drugs with existing patent-expired therapeutic compounds in order to produce a better therapeutic effect on a targeted indication.
Cannabinoids are compounds found in the Cannabis sativa (marijuana) plant. They interact with the endocannabinoid system in the human body, which plays a crucial role in regulating various physiological processes. The two primary cannabinoids are delta-9-tetrahydrocannabinol (“THC”) and cannabidiol (“CBD”). THC is the compound responsible for the psychoactive effects or the “high” associated with marijuana use. It has potential therapeutic applications, such as pain relief, nausea reduction (particularly in cancer patients undergoing chemotherapy), and appetite stimulation (especially in HIV/AIDS patients). CBD, on the other hand, does not produce a “high” and is not intoxicating. CBD has potential therapeutic benefits, specifically in anti-inflammatory, anti-anxiety, and antipsychotic therapeutics. It is also used in the treatment of epilepsy (e.g., Epidiolex, an FDA-approved CBD-based medication for certain types of seizures).
There are other Cannabinoids like Cannabigerol (“CBG”) and Cannabinol (“CBN”). CBG has anti-inflammatory and antioxidant properties and is being studied for its potential in treating conditions like inflammatory bowel disease and glaucoma. CBN may have sedative effects and is being explored for its potential as a sleep aid.
IXHL’s pipeline looks like this:
IXHL PIPELINE (IXHL WEBSITE)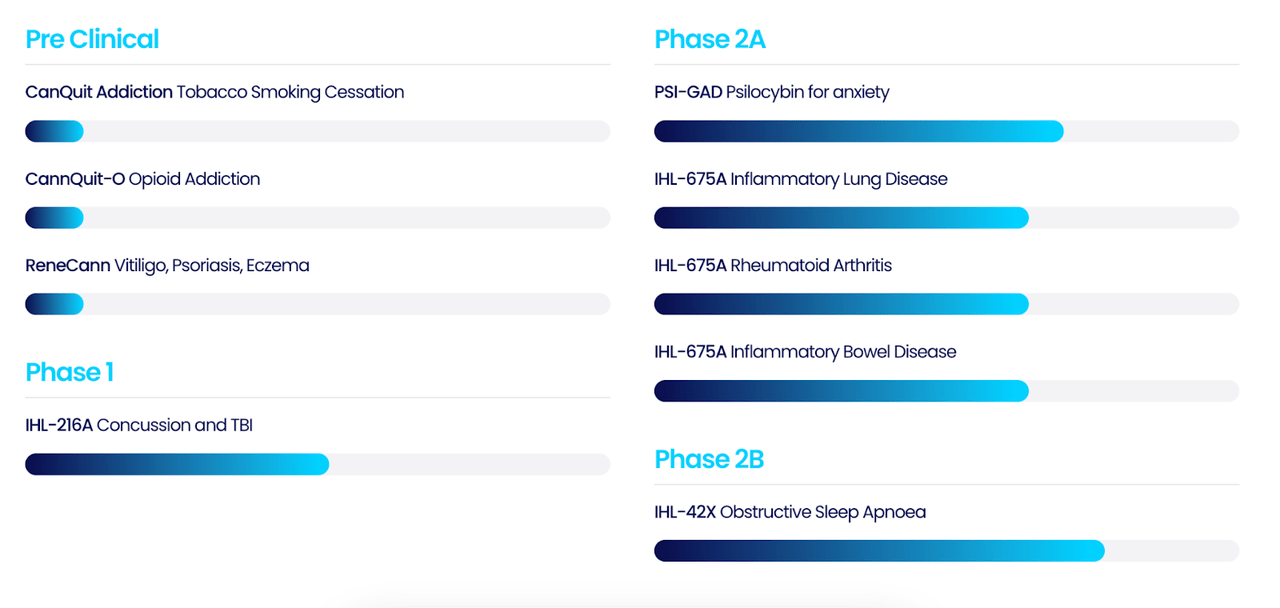
That’s a lot of pipeline, however the only ones with completed clinical trials are – IHL-42X in Obstructive Sleep Apnoea, MedChew™ Dronabinol in Nausea and Vomiting in Chemotherapy, CanChew Plus Gastro in IBS, APIRx-1601 Skin in Vitiligo, APIRx-1602 Skin in Psoriasis, and APIRx-1603 Skin in Atopic Dermatitis. Of these, IHL-42X appears to be the lead candidate, having completed a phase 2a trial and currently running a phase 2b (however, see below). A second candidate, CanChew Plus Gastro in IBS, has the same status. MedChew™ Dronabinol has completed a phase 1a trial and is now running a phase 1b trial in Nausea and Vomiting in Chemotherapy. The other programs read a little strange, more than one of them saying they have completed a higher stage trial and are now running, or contemplating, a lower stage 1. For example, APIRx-1601 says it has a phase 2 trial under its belt, but now it says it is running a phase 1 trial.
The rest of the pipeline is avowedly preclinical.
The lead program is OSA, where patients have interrupted breathing while sleeping. There are no approved “pharmacotherapies” in the U.S. IHL-42X, the lead candidate, consists of Dronabinol, a cannabinoid, and acetazolamide, a patent-expired approved drug. Dronabinol:
“binds to cannabinoid receptors, modulates signaling, and activates muscles that dilate the airway, whereas acetazolamide induces metabolic acidosis which signals to the body that there is excess CO, in the blood, inducing the taking of a breath.”
On looking further, here’s how the clinical progress is described:
IXHL TRIAL (IXHL WEBSITE)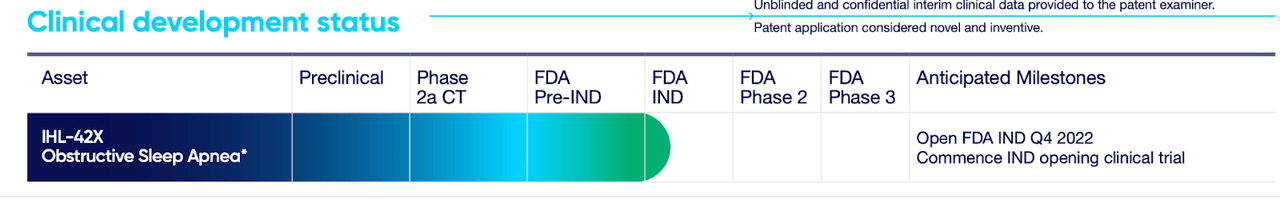
There’s a phase 2a CT (possibly clinical trial), but then there’s an FDA IND, which possibly means the phase 2a trial was held in another country and was not under the FDA’s jurisdiction. There’s no record on the official registry, either. On looking even further, the company presented data from this POC trial on its website; and although there’s no specific mention of a location, it does appear to be an Australian trial, given the CRO and other associations.
This data shows, inter alia, the following:
IXHL DATA (IXHL WEBSITE)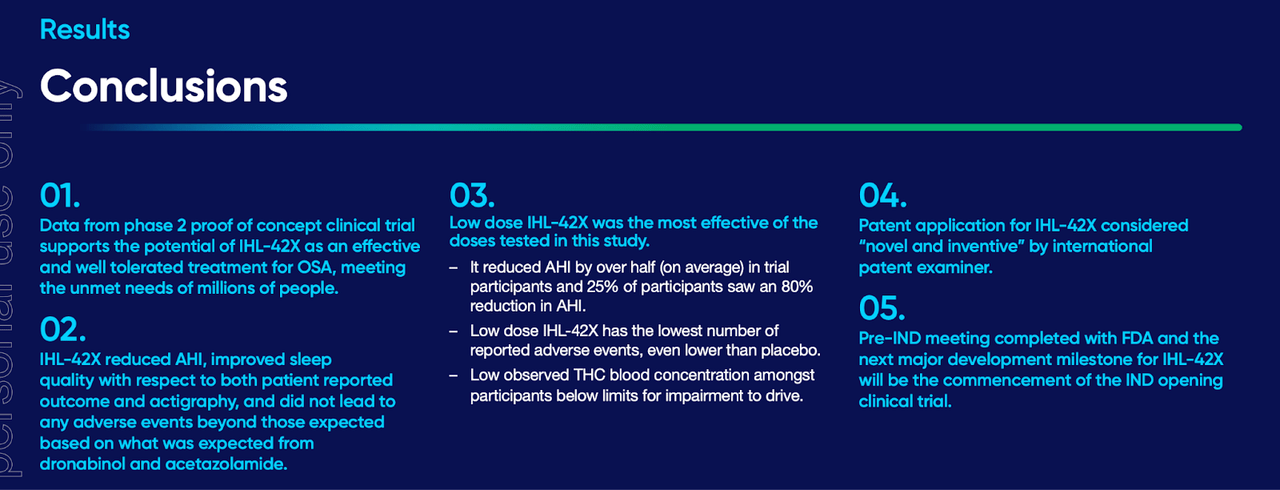
The company now has to do an FDA-jurisdictional trial, for which it received an IND in August. The company moved out of Australia and was listed on NASDAQ in July. I have no specific comment on the available data, and I will wait for U.S.-based trials for more clarity.
Finances
IXHL currently has a huge $1.8bn US market cap. According to Seeking Alpha, they have a cash balance of $22mn. The Corporate Presentation available on their website seems old and shows a market cap of $294mn and a cash position of AUD $37mn as of June 2022. These figures must have changed by now. This lack of data is a result of the fact that they completed their U.S. redomiciling only in November. So, we must wait for financial data.
They do have a 20F filing for the June quarter, which shows a cash balance of $33mn. Apparently, they spent $9mn on R&D, and total expenses are around $21mn. There are obvious discrepancies in these figures, and we need to wait for U.S. measures to kick in before we get more clarity.
Bottom Line
Incannex Healthcare Inc. has a novel idea of mixing cannabinoid molecules with patent-expired approved products. They have a long pipeline of sorts, and there’s some positive data, although not from a U.S. trial. I will add this to my very long list of “may become interesting” companies, and wait for more developments.