Bill Oxford
CASGEVY approval
CASGEVY is a groundbreaking gene therapy, owned by CRISPR Therapeutics AG (CRSP) and Vertex (VRTX) specifically designed for the treatment of sickle cell disease (SCD) in individuals aged 12 and older who experience frequent vaso-occlusive crises (VOCs).
Developed through a collaboration between Vertex Pharmaceuticals and CRISPR Therapeutics, CASGEVY stands out as the first FDA-approved therapy utilizing CRISPR/Cas9.
The primary issue in sickle cell disease is a mutation in hemoglobin, the protein in red blood cells responsible for oxygen delivery.
This mutation causes the cells to adopt a crescent or “sickle” shape, leading to restricted blood flow and reduced oxygen delivery, resulting in severe pain and potential organ damage.
The condition, predominantly affecting African Americans, is a significant health concern, impacting around 100,000 people in the U.S.
CASGEVY’s approach involves modifying the patient’s own hematopoietic (blood) stem cells using CRISPR/Cas9 technology. This gene-editing tool is directed to cut DNA in specific areas, allowing for precise DNA editing.
The edited blood stem cells, once reintroduced into the patient, engraft within the bone marrow and enhance the production of fetal hemoglobin (HbF).
This type of hemoglobin, prevalent during fetal development, is more effective than its adult counterpart and can prevent the sickling of red blood cells in SCD patients.
The treatment process is comprehensive and involves several steps. Initially, a mobilization medicine is administered to move blood stem cells from the bone marrow into the bloodstream.
These cells are then collected and sent to a manufacturing site to create CASGEVY. This phase can take up to six months. Following this, patients undergo a conditioning regimen in the hospital to prepare their bone marrow for the modified cells.
The CASGEVY infusion is then administered intravenously. Post-infusion, patients remain hospitalized for close monitoring, which can last from four to six weeks.
The most common side effects of CASGEVY include low levels of platelets and white blood cells, which can lead to increased risks of bleeding and infection. Patients are advised not to donate blood, organs, tissues, or cells after receiving the treatment.
That happens because in the process of preparing a patient for CASGEVY treatment, a step known as myeloablative conditioning is used, which is similar to chemotherapy.
This conditioning process involves using high-dose chemotherapy to clear cells from the bone marrow to make space for the modified stem cells to be transplanted.
This step is crucial for the success of the gene therapy but also brings some of the risks and side effects typically associated with chemotherapy, such as a temporary decrease in blood cell counts leading to increased risk of infections and bleeding.
In the short term improving the conditioning agent will be critical
Improving the conditioning agent used in the treatment process for therapies like CASGEVY could unlock significant advancements.
The conditioning process, particularly when it involves chemotherapy-like protocols, is a critical step in preparing a patient’s body for the acceptance and integration of genetically modified cells.
A gentler conditioning agent could potentially make the therapy accessible to a broader range of patients. Currently, the intensity of the conditioning regimen limits the eligibility of patients, particularly those who may not be able to tolerate the harsh effects of chemotherapy-like treatments.
A less toxic conditioning regimen could be safer for a wider range of patients, including those with comorbidities or those who are in a weaker health state.
The toxicity associated with current conditioning methods can lead to a range of side effects, from infections to organ damage. A more targeted and less toxic conditioning agent could reduce these risks, leading to a safer overall treatment experience.
Goes without saying that by minimizing the potential for complications during the conditioning phase, the overall success rates of gene therapy treatments like CASGEVY could be improved. Successful engraftment of modified cells is crucial for the therapy’s effectiveness, and a gentler conditioning process could enhance this aspect.
Reducing the intensity and toxicity of the conditioning regimen could also lead to a better quality of life for patients both during and after treatment. This includes a shorter recovery time and less time spent managing the side effects of conditioning.
At the JP Morgan Healthcare conference, Sam Kulkarni (CRISPR Therapeutics CEO), said the company is working on a new conditioning agent that might unlock the therapy to triple of the current eligible population.
Evolution in the medium to long term
In the medium to long term, the innovation leap will be to focus on developing in vivo editing techniques for hematopoietic stem cells (HSCs) within the bone marrow.
The goal is to create delivery systems that can edit these HSCs directly in the patient’s body (“in situ”). In the JP Morgan conference, CRISPR’s CEO stated that this approach aims to make treatment accessible to the approximately 1 million sickle cell patients worldwide who cannot afford the current form of CASGEVY.
This also unlocks other therapeutical applications. For instance, the development of highly sophisticated CAR-T (Chimeric Antigen Receptor T-cell) therapies targeting CD19 and CD70.
These CAR-T cells have been engineered with multiple genetic modifications to enhance their effectiveness. CAR-T therapy is a form of immunotherapy where a patient’s T-cells are modified to recognize and attack cancer cells.
The reference to CD19 positive lymphoma indicates the potential use of these engineered CAR-T cells in treating certain types of lymphoma.
Additionally, these CAR-T cells hold promise not just for cancer treatment but also for autoimmune diseases, where the immune system mistakenly attacks the body’s own cells. That also opens the door to improvements in organ transplant.
Broader applications
The evolution from CTX110 to CTX112 in CAR-T therapy primarily revolves around advancements in genetic engineering.
CTX110, the first-generation allogeneic CAR-T therapy, demonstrated the feasibility and effectiveness of using healthy donor-derived T cells to treat lymphoma, showing durable responses.
The progression to CTX112 involves additional genetic edits, aiming to enhance the therapy’s efficacy.
CTX110 and CTX112, as allogeneic CAR-T therapies, use “off-the-shelf” cells. This approach involves using T-cells from healthy donors rather than the patient’s own cells, which is the case in autologous CAR-T therapy.
Allogeneic CAR-T cells are genetically engineered to be compatible with multiple patients, making the treatment more readily available and potentially reducing manufacturing time and cost compared to personalized autologous CAR-T therapies.
This “off-the-shelf” approach represents a significant advancement in making CAR-T cell therapies more accessible to a broader patient population.
As mentioned, it also opens the door to other CAR-T therapies, particularly allogeneic CD19 directed CAR-Ts, for treating autoimmune diseases.
The idea is that CAR-Ts can reset the immune system by depleting B-cells, potentially curing the disease. Their allogeneic CAR-T (CTX110) also shows similar B-cell depletion effects in oncology trials.
In vivo platform for scaling and lower costs
In vivo revolves around a “plug and play” approach for gene editing, using lipid nanoparticles (LNPs) to deliver edits to the liver.
They’ve achieved significant editing efficacy in preclinical studies, specifically in non-human primates (NHPs), demonstrating around 70% liver editing, which implies effective targeting of hepatocytes. The initial clinical targets include ANGPTL3 and LPA, along with other targets like PCSK9.
This approach is seen as paradigm-changing, potentially offering a one-time injection solution for lifelong management of conditions like high cholesterol. The goal is to emulate natural loss-of-function mutations found in some people who naturally have lower cholesterol and cardiovascular risk.
This methodology, similar to that used in developing CASGEVY, aims to replicate beneficial genetic variations found in nature using CRISPR technology. The first of these targets, CTX310, is already in clinical trials, targeting ANGPTL3 to reduce triglycerides and cardiovascular disease risk.
Another example is the CTX211 program, now solely owned by CRISPR Therapeutics after Vertex Pharmaceuticals’ subsidiary ViaCyte opted out, is an investigational therapy for Type 1 Diabetes (T1D).
CTX211 is an allogeneic, gene-edited, stem cell-derived therapy aimed at enhancing cell fitness. This therapy is designed to be immune-evasive and helps patients produce their own insulin in response to glucose, offering a potentially transformative approach to treating T1D.
This program is part of CRISPR Therapeutics’ diverse portfolio, which includes therapies for various diseases, utilizing CRISPR/Cas9 gene-editing technology.
Scaling commercial adoption
One of the critical aspects to achieve broader commercial adoption is to do it at a reasonable cost.
There is a big difference between production for clinical tests and production for commercial applications. For that to happen, costs of goods sold need to go down with production.
One example is the COGS for CTX001. The lower cost of goods (COGS) at CRISPR Therapeutics’ manufacturing facility in Framingham, Massachusetts, is attributed to several factors.
The autologous nature of their gene-edited stem cell candidate CTX001 allows for relatively easy scaling from clinical to commercial production. This is achieved by adding more suites to the facility, which means increasing throughput without significant changes to the biochemistry of the process.
The production of CTX001 involves collecting and editing a patient’s blood stem cells to increase fetal hemoglobin expression, which is scaled up using contract manufacturing organizations.
The facility’s design enables efficient scaling, supporting the company’s various investigational therapies while adhering to regulatory guidelines.
This approach to manufacturing provides CRISPR Therapeutics with the flexibility and capacity needed for rapid clinical trial progression and global distribution of their therapies.
TL;DR
I have already written extensively about CRISPR Therapeutics AG. In my previous pieces, I have mostly focused on their CASGEVY therapy, which I have previously valued at $7 billion.
Nevertheless, my point here was to showcase, how the CASGEVY launch unlocks and validates the potential on the company’s pipeline. As we have seen there is a lot in there.
The company has a broad portfolio in gene editing, spanning hemoglobinopathies, immuno-oncology, autoimmune diseases, in vivo Type I diabetes, and other areas.
With a strong financial foundation, they have multiple clinical trials and developments underway.
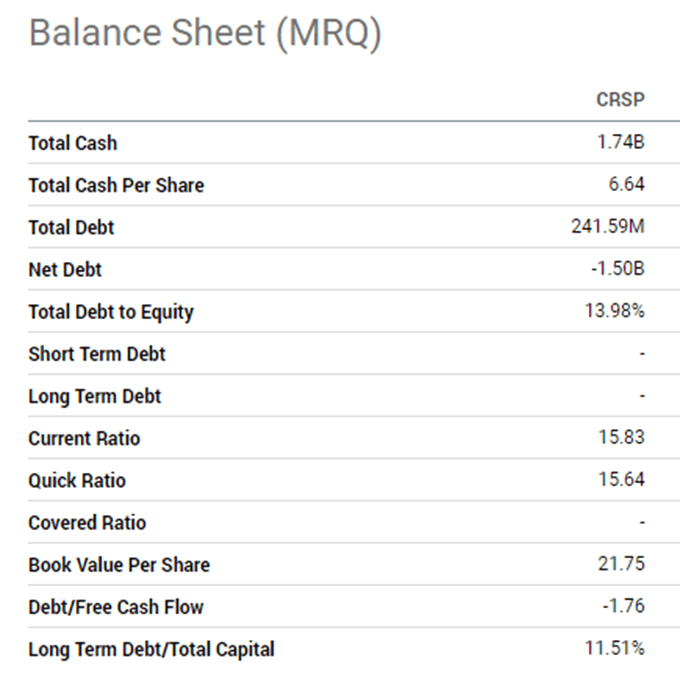
Source: Seeking Alpha
For 2024, they anticipate significant clinical outcomes from various assets, reflecting the company’s leading position in CRISPR-based medicine and its commitment to expanding its impact on numerous medical conditions.
The previous paragraphs are meant to provide some color on the short, medium and long term for CRISPR AG, but also for the whole space. The CRISPR/Cas9 discovery unlocked tremendous innovations. CASGEVY is just the beginning.
Disclosure: LONG CRSP. This text expresses the views of the author as of the date indicated and such views are subject to change without notice. The author has no duty or obligation to update the information contained herein. Further, wherever there is the potential for profit there is also the possibility of loss. Additionally, the present article is being made available for educational purposes only and should not be used for any other purpose. The information contained herein does not constitute and should not be construed as an offering of advisory services or an offer to sell or solicitation to buy any securities or related financial instruments in any jurisdiction. Some information and data contained herein concerning economic trends and performance is based on or derived from information provided by independent third-party sources. The author trusts that the sources from which such information has been obtained are reliable; however, it cannot guarantee the accuracy of such information and has not independently verified the accuracy or completeness of such information or the assumptions on which such information is based.
Sources:
CRISPR Therapeutics Highlights Strategic Priorities and 2024 Outlook
CRISPR Therapeutics Highlights Strategic Priorities and 2024 Outlook
FDA Approves First Gene Therapies to Treat Patients with Sickle Cell Disease
Casgevy: Uses, Dosage, Side Effects, Warnings – Drugs.com
Vertex and CRISPR Therapeutics Announce US FDA Approval of CASGEVY™…
CRISPR Therapeutics AG (CRSP) 42nd Annual J.P. Morgan Healthcare Conference (Transcript)
Crispr Is Getting A Foot In The Door, And Maybe Investors Should Too (NASDAQ:CRSP)
Beyond Crispr’s Exa-Cel Program: A Scalable Pipeline (CRSP) CRISPR (CRSP) And Vertex (VRTX): ICER Sheds Light On The Future Of Sickle Cell Treatment
Original Post
Editor’s Note: The summary bullets for this article were chosen by Seeking Alpha editors.

