AlexSava
Sometimes deviation is merely a stage in progression. COMPASS Pathways plc (NASDAQ:CMPS) research exemplifies that notion. CMPS is a mental healthcare company that specializes in psilocybin therapy. It’s produced the two largest psilocybin trials ever recorded and has made remarkable discoveries regarding the effects of psilocybin. CMPS is presently in phase 3 trials for Treatment-Resistant Depression (TRD), and phase 2 trials for both PTSD and Anorexia Nervosa.
I believe CMPS will most likely be the winning steed in the psychedelics race due to the rapid pace of its research. It appears CMPS is on track to receive FDA approval for TRD treatment via psilocybin before any of its competitors, so it will benefit from this first-to-market opportunity. However, I believe CMPS is a hold right now due to the risks it faces on the path to commercialization.
Psilocybin and Neurological Health
How does psilocybin affect the brain? Aside from hallucinations and sensory changes, psilocybin affects the brain by improving neuroplasticity – the brain’s ability to reorganize itself. Basically, it makes the brain malleable, and ripe for molding. A plethora of research on rats has already demonstrated a correlation between improved cognitive function and acute doses of psilocybin. Furthermore, some of these studies provided conclusive evidence of adult neurogenesis in the hippocampus. Neurogenesis is a process in which the brain produces new neurons, while others are repaired. From the foundation of these studies and many others, psilocybin has been used in trials to treat many mental illnesses.
Market for Psilocybin
As such, CMPS is currently in trials for treating treatment-resistant depression, PTSD, and Anorexia Nervosa with psilocybin. Currently, it is in phase 3 trials for TRD which are expected to conclude in 2025. After the third phase, CMPS is likely to submit an NDA to seek FDA approval for market use.
Yet, the court of public opinion is divisive. According to a 2021 poll, 65% of American voters do not believe that psilocybin is appropriate for medical use. However, popularity for psilocybin rose in recent years due to a renewed interest in psychedelic therapy. According to a poll by Delic Holdings and Harris, 65% of Americans with mental health issues want access to psychedelics.
Furthermore, VA backed research for treating PTSD using psilocybin has led to positive coverage in various news outlets and increased its popularity among veterans. As is, Australia is planning to legalize MDMA and psilocybin treatment on the first of July 2023, which could also influence public perception as Canada’s legalization of medical marijuana did in 2001.
Company Presentation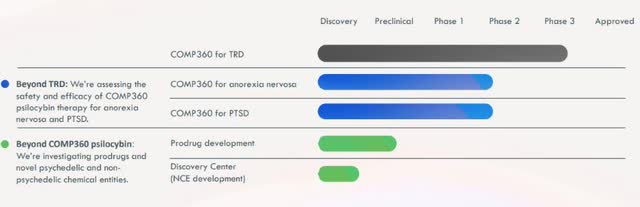
Treatment Resistant Depression Trials
Of the 8.9 million adults in the US suffering from depression, 30% suffer from TRD. Given depression’s prevalence, the global anti-depressants market is valued at $13.5 billion and is projected to grow 7.2% by 2027. I believe if COMP360 is FDA-approved, the treatment could capture a sizable chunk of the market thanks to psilocybin’s efficiency.
CMPS’s phase 2b trial concerning treatment-resistant depression provided promising results. During the second phase of its trial, 30% of participants who received the 25 mg dosage of COMP360 psilocybin along with therapy experienced a significant rate of remission. And 25% experienced a sustained remission for 12 weeks. At present, CMPS is in its third phase of TRD clinical trials, which are set to conclude in March 2025.
The sustained response of psilocybin is a competitive advantage COMPS360 has over other TRD treatments. According to a study by Dr. Makasi Kato 60%-70% of TRD patients do not experience remission, which is why sustained remission is a major accomplishment for COMP360.
Since TRD doesn’t respond to traditional therapeutics, psychiatrists tend to prescribe multiple medications such as antidepressants, antipsychotics, thyroid hormones, and mood stabilizers to tackle TRD. These augmentation drug cocktails pose significant costs and complications for patients.
Considering that TRD is the hardest form of depression to treat and has the highest mortality rate, CMPS’s progression to the third phase of trials – the largest of its kind – is an indication of its treatment’s potential.
Company Presentation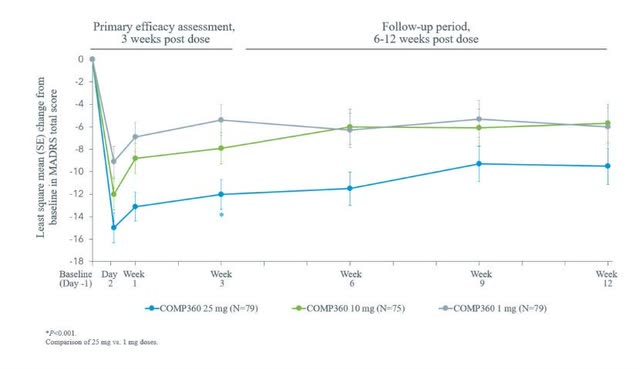
PTSD Trials
Presently, CMPS is conducting phase 2 clinical trials for PTSD which is synonymous with a heightened threat response. This translates to a lot of activity in the amygdala. Psilocybin decreases activity in the amygdala while promoting neuroplasticity and neurogenesis in the hippocampus, in turn allowing patients to process and overcome trauma. Currently, CMPS is expected to conclude its phase 2 trial in August of this year.
The market for an effective PTSD treatment is significant since 7 out of every 100 Veterans will have PTSD at some point in their lifetime. According to the VA, its prevalence is slightly lower among civilians since the VA found 6 out of every 100 adults will have PTSD in their lifetime. Given that 7% of veterans have PTSD, the VA has focused its efforts on finding solutions which is why the VA’s trials on psilocybin could be a deciding factor in the push for medical legalization.
As is, PTSD treatments are usually also anti-anxiety and antidepressant medications. Some prominent examples are Paroxetine, Zoloft, and Paxil. These drugs are SSRI inhibitors, which modulate serotonin in the brain. While Zoloft is a medication used to treat a variety of conditions, before its patent expired in 2007 it made an average of $3 billion annually. As is, the market for PTSD in the US is valued at $750.3 million, which is an especially large portion of the $2.5 billion global market. Therefore, I believe that CMPS’ trials treating PTSD with COMP360 are especially well-timed and if its Phase 2 trials reveal promising results later this year, it could enter its third phase.
Anorexia Nervosa Trials
CMPS is also conducting phase 2 trials for Anorexia Nervosa, which is expected to end in December of this year. Anorexia Nervosa is a deadly mental illness with no effective treatment. Two central aspects of Anorexia Nervosa are cognitive inflexibility and serotonergic signaling, which is a fancy way of saying issues managing serotonin. Psilocybin treats cognitive inflexibility through neuroplasticity and also modulates serotonin.
Anorexia Nervosa is a hard to treat condition which affects 2.2% of women, and 0.3% of men. Currently, there are two medications that are commonly used for treating Anorexia Nervosa – olanzapine and cyproheptadine – neither of which are FDA approved for treating Anorexia Nervosa.
One reason there have not been any FDA approved treatments for Anorexia Nervosa is because acquiring a large sample size is tricky since the trial’s success is measured in terms of patients’ weight gain. However, CMPS may not face this issue because it has access to multiple testing locations all over the world which could be used simultaneously during the upcoming Anorexia trials. While its sample size will likely not equal the TRD phase 2b trial, getting an average sample size for an Anorexia Nervosa trial is more than adequate.
If the trial is successful, it could be perceived as a medical breakthrough because both olanzapine and cyproheptadine are treatments intended for treating other conditions. Additionally, they treat the symptoms without addressing the underlying issue of cognitive inflexibility.
In the case of antipsychotics such as olanzapine, it can promote neuroplasticity, however not as efficiently as psilocybin. Therefore, psilocybin would likely be a more effective treatment for Anorexia Nervosa, potentially offering sustained improvement. If sustained improvements are documented over a long period of time, then the FDA may provide it with orphan drug status, which would accelerate the approval process.
Off Label Use
Once COMP360 is legalized as an FDA-approved drug for a single use, then off-label use is possible as well. Off-label use may at first seem like a mischievous endeavor, but it’s actually not what some of you have in mind.
Off-label use in this context refers to the ability of a physician to prescribe a medication for uses the FDA has not approved as long as there is a medical basis for their decision. If COMP360 is approved for TRD, it could be prescribed for other treatments as long as there is a precedent for it. However, legal ramifications from malpractice lawsuits concerning off-label use mean doctors would be wary of prescribing psilocybin unless there is substantial evidence or trials backing their decision.
As is, off-label use drastically expands the potential uses of COMP360 since there is a medical basis for using psilocybin to treat: addiction, strokes, traumatic brain injuries, and possibly even autism. As research accumulates and the FDA potentially approves other psilocybin drugs, I believe COMP360 would be positioned at the forefront if it is the first to market. Then, with time, COMP360 would be able to enter an increasing number of markets thanks to off-label use.
Competition
Currently, I believe that CMPS is unmatched in its likelihood of receiving FDA approval. Since COMP360 is the only psilocybin based drug in the third phase of the FDA approval process, it has an outsize advantage over its competition.
There are 10 psilocybin based trials in phase 2 aside from CMPS’s two pending trials for PTSD and Anorexia Nervosa. The companies leading these 10 trials are: B.More, APEX Labs, Nova Mentis, Usona, Braxia Scientific Corp. (OTCPK:BRAXF), Incannex, Cybin (CYBN), Clairvoyant, Ceruvia, and Psyence. Overall they are targeting forms of depression, bipolar disorder, and PTSD in their trials while CMPS is the only company pursuing Anorexia Nervosa treatment in its psilocybin trials. So far none of these companies have had more than one psilocybin trial in the second phase which is another sign that CMPS is the leader in this space.
As is, CMPS has 4 public competitors: Braxia Scientific Corp., Nova Mentis Life Science Corp (NOVA), Opitimi Health Corp. (OPTI), and Cybin Inc. Compared to its competitors, CMPS provides a larger quantity of psilocybin research, with a more sizable quantity of participants.
Unlike some of its competitors, CMPS has secured the backing of Atai Life Sciences (ATAI), a clinical-stage biopharmaceutical company which owns a 22.4% stake in CMPS. Peter Theil’s Founder’s Fund also owns a .62% stake in CMPS while Ark Investment recently raised its stake to 5.6%.
Cash Runway
As is, CMPS has a high burn rate which makes a capital raise likely. The company believes its cash balance of $143.2 million will fund capex for at least the next twelve months. This is in line with its FY 2023 guidance of operating activities costing between $85 and $110 million. Given the scale of its Phase 3 trials, it will probably reach the upper end of this guidance if not exceed it.
| Year | R&D | Total Operating Expense | % Change | Cash and cash equivalents | % Change |
| 2022 | 65,053,000 | 110,403,000 | 32.60% | 143,206,000 | -47% |
| 2021 | 44,027,000 | 83,221,000 | 61% | 273,243,000 | 43% |
| 2020 | 23,366,000 | 51,393,000 | 142.60% | 190,327,000 | 662% |
| 2019 | 12,563,000 | 21,179,000 | – | 24,966,000 | – |
As annual R&D expenses have increased over the last 4 years, so have CMPS’s operating expenses. The company’s cash position has declined roughly 16.25% each quarter.
| Quarter | Loss from Operations | Cash and cash equivalents | % Change in Cash |
| Q1 2023 | 31,788,000 | 117,103,000 | -18% |
| Q4 2022 | 32,191,000 | 143,206,000 | -17% |
| Q3 20222 | 25,536,000 | 173,076,000 | -16% |
| Q2 2022 | 27,256,000 | 207,177,000 | -14% |
| Q1 2022 | 25,420,000 | 243,684,000 | – |
The company has historically derived funding from the sale of convertible preferred shares, convertible loan notes and ADSs in its IPO and Follow-On Offering. CMPS may use positive trial results as an opportunity to raise funding and with data from its phase 2 PTSD study is expected in late 2023, a capital raise may be around the corner. This capital would ideally fund CMPS’ operations until the final Phase 3 results of its TRD trials are released.
Risks
As a pre-product, clinical-stage mental health care company, CMPS faces some significant risks.
The most notable risk is that CMPS may not pass phase 3 trials. Approximately 33% of drugs pass the third phase of FDA approval, if COMP360 does not it would likely devastate the company. The FDA approval process is also very rigorous and time consuming. Issues may arise in the trials that result in its postponement, fiscally straining CMPS. If they were to extend their timeline as a result, the company would also need to raise more capital.
On this note, during COMP360’s review process and prior to any approval the FDA or other regulatory bodies may require additional data regarding its abuse or misuse potential which may also delay approval.
These sorts of delays could potentially allow other synthetic psilocybin alternatives such as TRP-8802 and NM 1001 to gain approval before COMP 360 – stealing its first to market advantage.
Meanwhile, CMPS has incurred significant losses since its inception and the company expects these losses to continue for the foreseeable future. For this reason, CMPS needs substantial additional funding to complete the development and commercialization of its investigational COMP360 psilocybin therapy.
If such funding is not available, this may force CMPS to cut back on its R&D, sell the rights to certain IP, or raise capital through dilution. Shares outstanding reportedly grew 7.9% last year and could continue to grow depending on the company’s funding needs.
Even if COMP360 is approved for treating TRD, the negative stigma and issues related to psilocybin’s status as a controlled substance could hurt demand. Negative news coverage could also exacerbate fears regarding psilocybin’s potential side effects which include psychotic episodes, paranoia, anxiety, and other effects.
CMPS Stock Forecast
TradingView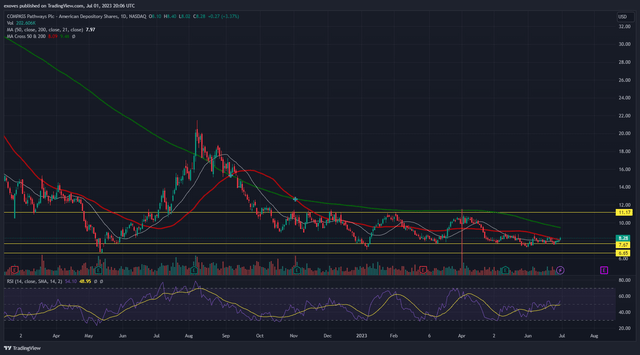
CMPS is trading in a sideways channel between $7.67 and $11.17 and will likely continue to trade in this channel until a strong catalyst pushes it out. Barring any unexpected changes in the political agenda, CMPS has a few upcoming catalysts. The results of its phase 2 PTSD trials could be released as soon as August and its phase 2 trials for Anorexia Nervosa are expected to conclude in December.
Conclusion
While these binary events could help push CMPS out of its channel, at this time I do not see a justification for the risk CMPS presents. Given that the company will need to raise capital soon, I will hold off on giving CMPS a buy rating and wait for the results of its Phase 2 trials. If patients show a sustained, substantial improvement, COMP360 could be given breakthrough therapy status for treating Anorexia and PTSD.
While the company is clearly a promising pioneer in psilocybin treatment, there is still a long road to commercialization ahead. Issues with treatment administration, adoption, and medical legalization could continue to affect the company’s operations even if COMP360 is FDA approved.