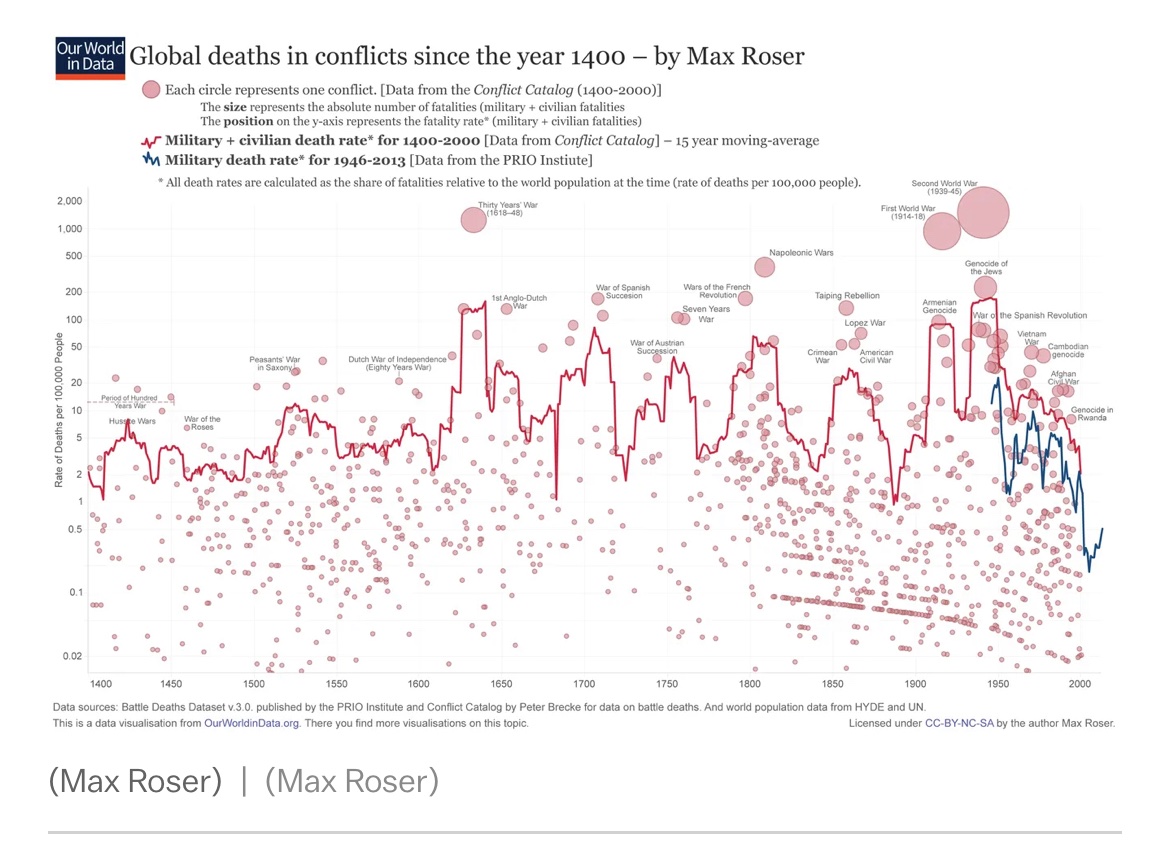
Just over 50 years ago, President Richard M. Nixon signed into law the National Cancer Act of 1971, which established the National Cancer Institute. Cancer had become the second leading (after heart disease) cause of death in the US in 1970. The National Cancer Institute (NCI) from its establishment had broad powers, including the creation of new cancer centers, researcher and training programs, the granting of research grants, and the establishment of fifteen new cancer research centers. President Nixon termed the establishment of the NCI as a declaration of war on cancer.
That terminology has portrayed the quest for effective cancer prevention and treatment into something like what took place after the abrogation of the Molotov-von Ribbentrop pact, which precipitated World War II. But “cancer” should not be compared with Hitler’s invading armies. Cancers are more like criminal enterprises of many different kinds, only related to one another in that a drug-pusher might become a burglar, and a blackmailer could also be a swindler. And any of them, depending on the circumstances, might commit murder.
Cancers are usually grouped as to the part of the human body they attack, viz., breast cancer, lung cancer, prostate cancer. But as it became evident that each cancer was essentially different, it became necessary to adopt a more precise terminology. This Doc Gumshoe installment will discuss new treatment options for KRAS G12c mutant advanced solid tumors, peripheral T-cell lymphomas, non-small cell lung cancer, biliary tract cancer, PD-1/L1 stomach cancer, metastatic castration-resistant prostate cancer, and non-metastatic hormone-sensitive prostate cancer, among others.
That’s just a short list of some of the many distinct kinds of cancer that are out there as threats. Listing them that way may help us understand that cancer is not a single entity, but a large loosely-connected constellation of threats that have to be understood individually, and can most effectively be targeted if they are targeted individually.
Traditional cancer therapy: a short summary
Targeting individual cancers represents a distinctly new phase in cancer treatment. For most of the history of cancer treatment, there were essentially three approaches, which are still widely used: surgery, radiation, and chemotherapy. We’ll say a few words about each one.
Surgery
If the patient has a solid tumor which has not yet metastasized to other parts of the body, surgery is intuitively the most direct and effective approach: remove the tumor and the margins around the tumor, where some cancerous cells may be lurking. In some cases, this leads to complete remission without the need for further therapy of any kind; in some cases surgery is followed by some forms of chemotherapy. This approach to treatment has produced five-year survival rates of about 90% in breast cancer patients (for localized breast cancer the five-year survival rates are close to 100%, and for regional breast cancer about 80%), and higher than 95% in prostate cancer patients.
Improvements in surgery for cancer have largely been the result of great advances in imaging techniques, which permit surgeons more accurately to locate and remove cancerous tumors. We should also not neglect the possibility that the surgeons themselves are becoming more skillful.
Radiation
Radiation therapy has also benefitted enormously by improvements in imaging, permitting external beam radiation to pass through the target tumor precisely. Radiation therapy is now typically carried out in such a way that the beam of radiation passes through the patient’s body in different paths, so that only the target tumor is repeatedly exposed to radiation. This is accomplished by changing the position of the patient’s body with relation to the source of the radiation.
Radiation can also be delivered in the form of beads of radioactive material which can be directed to the site of the cancer. SIR Spheres (selective internal radiation therapy microspheres) from SIRTeX Medical Ltd, and TheraSpheres, from BTG International, are tiny radioactive spheres, containing yttrium90, which are conveyed to the liver via small hepatic arteries. The delivery of radiation by means of these microscopic beads, rather than by needle implantation, as in prostate cancer, is a fairly recent approach to getting therapy right to the cancer site. This tactic takes advantage of the propensity of tumors to foster the growth of blood vessels that bring the nutrients the cancer cells need to grow and multiply.
Chemotherapy
Chemotherapy consists of dosing the patient with a class of drugs that are to some degree toxic, but that are more readily taken up by the cancer cells than by the rest of the body’s cells. The reason those drugs work – to the degree that they do work – is that by and large cancer cells grow faster and absorb nutrients more quickly than the non-cancerous cells, so the cancer cells essentially drink more of the poison. Those drugs are of course affected by many side effects, perhaps the most concerning of which is neutropenia, which is a deficiency in the number of white blood cells in the patient’s circulation, leading to a reduced capacity to combat infection. The most commonly-known side effects are nausea and vomiting, and, in particular, hair loss. But chemotherapy can also produce problems with cognition and short-term memory.
Among the most commonly-used chemotherapy agents are drugs that are also used to treat a number of other diseases and conditions. Methotrexate was the first drug used to treat any form of cancer. It was employed in 1956 to treat a rare form of cancer called choriocarcinoma. It is also used to treat some autoimmune diseases such as rheumatoid arthritis and psoriasis in the early stages. Prednisone is used to manage inflammation and in some cases to treat the common cold. Cyclophosphamide is sometimes used to treat kidney diseases.
There are several classes of chemotherapy drugs. Alkylating agents such as carboplatin and cisplatin disrupt the DNA in cells and prevent them from multiplying. Anti-metabolites such as fluorouracil and gemcitabine are mistakenly ingested by cancer cells as though they were nutrients, but what they do is prevent the cells from taking in nutrients so that the cells starve to death. Plant alkaloids prevent cells from dividing and reproducing; examples are vincrisine, paclitaxel, docetaxel, and irinotecan. Anti-tumor antibiotics cause DNA strands to unravel, which prevents the cells from dividing and reproducing; examples include doxorubicin and daunorubicin.
Doctors treating cancer patients frequently combine drugs from these classes in order to attack the cancer cells through several mechanisms of action. And they attempt to use lower dosages of individual drugs, thereby dialing down the damage to the non-cancerous cells. However, with chemotherapy there is always some damage to non-cancerous cells.
… but some essential chemotherapy drugs are experiencing shortages
Drugs used in chemotherapy are mostly generic drugs, which means that they are no longer under patent. The pharmaceutical company that originally developed these drugs may sometimes continue to make the drug, but in most cases generic drugs are made by companies devoted to manufacturing generic drugs – companies that had nothing to do with the development of the specific drug, nor of any drug. The emphasis is on economy. Most of these generic drug manufacturing companies are in less developed parts of the world, and they are subject to a range of stresses – economic, political, and stresses resulting from local events. As a result, the supply is not reliable, resulting in some cases in interruption of treatment.
In past months, there have been severe shortages of carboplatin and cisplatin, with the result that oncologists had difficult choices with regard to the treatment regimens of some patients. In some cases, they had to space the dosage schedule at longer intervals, as the supply of the drugs trickled down. And in some cases, the only choice was to substitute a significantly more expensive agent that is still under patent. Either way, there could be harm to the patient.
Drug shortages affect the whole range of patients that depend on generics for regular treatment. Industry observers note that about 130 widely-used generics are subject to unreliable supply, as the generic manufacturers deal with uncertain conditions in their home countries.
Current cancer statistics
“reveal” emails? If not,
just click here…
The total number of cancer cases and deaths has been tallied most recently for the year 2020. The table below shows the estimated numbers for 2023.
The chief data sources for that table were the American Cancer Society and SEER (Surveillance, Epidemiology, and End Results, part of NIH). As you can see, some specific cancers of great interest don’t show up in the table, because they are lumped in with other cancers in the same general class. For example, prostate cancer, which is the single cancer most affecting men, is included in the genital system category. The actual data for prostate cancer (which the American Cancer Society does report if you dig a little deeper) is that the estimate for cases in 2023 is 288,300, which is second only to breast cancer, which, with 297,790 estimated cases is the most frequently-occurring single cancer. The deadliest cancer is pancreatic cancer, in which 50,550 deaths are projected to take place in an estimated 64,050 new cases.
Estimated five-year survival rates for these cancers run from 8.3% for pancreatic cancer to 97.4% for prostate cancer and 96.7% for thyroid cancer. Melanoma with an 89.4% five-year survival rate and breast cancer with an 88.7% five-year survival rate are not far behind. Other cancers with discouragingly low survival rates are liver and bile duct cancer (lumped in with the digestive system), with a five-year survival rate of 17.2%, and lung and bronchus cancer (in with respiratory system), with a five-year survival rate of 18.3%. All in all, the five-year survival rate for all cancer sites is about 66%. In other words, according to those figures, two thirds of patients diagnosed with cancer are still alive five years after diagnosis.
I should point out that predicting those five-year survival rates out to three decimal places is a bit presumptuous. Who knows how long the cancers are growing prior to the diagnosis? And who knows what the actual cause of death was in many of those individuals?
Another thing to take into consideration when we consider five-year survival rates is that those first five years are frequently only the beginning. Many cancer survivors live ten, fifteen, twenty years and even longer after the treatment that resolved their cancers.
The number of cancer deaths per 100,000 population has dropped considerably in the US since the turn of the current century, from about 200 in the year 2000 to about 140 currently. However, that particular statistic does little to capture the advances in cancer treatment. Cancer is second only to heart disease as the leading cause of death, and there have also been great advances in the treatment of heart disease. But no matter how effective the current treatment programs for these two killer diseases are, they do not confer immortality on the patients. A highly successful new cancer treatment might give patients several more years of life. However, they will ultimately show up in the mortality statistics, whether from cancer or other causes.
Enough about these statistics, which tell us something, but certainly do not give a complete picture of the current state of the cancer treatment arena.
Some recent advances in cancer treatment
Many of these were announced at the recent gathering of the American Society of Clinical Oncology (ASCO), which was held the first week of June.
I should point out that a good deal of the news had to do with new drugs that led to improved outcomes in cancer types that had previously been resistant to most treatments. The usual process is to test the new drug in combination with whichever existing drug had led to the best outcomes, even if those outcomes in the particular cancer had been far from satisfactory. Significantly improved outcomes with the combination of the existing drug and the new candidate drug would then likely lead to FDA approval of the candidate drug for that specific cancer. Even if the specific cancer is rare and sales of the new drug for that cancer would not make a lot of money for the pharmaceutical company, drug makers eagerly pursue that kind of opportunity, because once their new drug gets the FDA blessing, it is likely that the new drug would turn out to be helpful in other cancers, leading to more FDA approvals. That’s the process that many of the current cancer blockbuster drugs went through.
Competition among KRAS inhibitors
KRAS, in case you’re wondering, stands for “kirsten rat sarcoma viral oncogene homologue.” KRAS is a common protein which is particularly subject to mutation. The mutated forms of the protein are associated with a series of highly fatal cancers, so KRAS is considered an oncogene – a cancer-causing gene. These cancers include pancreatic ductal adenocarcinoma, non-small cell lung cancers (NSCLC) and colorectal cancer.
Mutated forms of other proteins are also oncogenes, such as epidermal growth factor receptor (EGFR) or anaplastic lymphoma gene fusion (ALK), and numerous clinical trials have found that directly targeting those genes significantly extended cancer-free survival in patients and were also much less toxic than standard chemotherapy regimens. However, despite about 40 years of effort, targeting the KRAS oncogene has been mostly unsuccessful. Cancers related to KRAS were considered essentially “undruggable.”
The KRAS protein functions as a closely-regulated molecular switch that controls multiple signaling cascades by cycling between activated and inactivated conformations. Mutations in the KRAS protein essentially freeze the switch in the “on” position, signaling cancer cells to grow and proliferate. One particular KRAS mutation, designated G12C, specifically triggers the growth of non small-cell lung cancers. Other KRAS mutations, G12D and G13C, favor the growth of other cancers.
After decades of frustration, two agents have been developed to address KRAS G12C. These are Lumakras (sotorasib), from Amgen, and Krazati (adagrasib) from Mirati Therapeutics. Lumakras was the first KRAS inhibitor on the market, and was initially received with high hopes. Disappointment set in when the initial data was released on Lumakras combination therapy with Merck’s Keytruda (a checkpoint inhibitor), as significant liver safety issues emerged. Depending on which other checkpoint inhibitors Lumakras was combined with, the liver toxicity rate climbed to over 30%, and in some cases reached as high as 60%.
The initial data on Krazati did not show similarly significant liver safety issues, giving Krazati an edge over Lumakras. However, neither drug demonstrated substantial efficacy advantages over Keytruda alone.
Keytruda alone, by the way, is FDA-approved in eight cancer forms: KRAS G12C mutant advanced solid tumors, peripheral T-cell lymphomas, non-small cell lung cancer, biliary tract cancer, PD-1/L1 stomach cancer, metastatic castration-resistant prostate cancer, non-metastatic hormone-sensitive prostate cancer, and metastatic liver cancer.
As recently announced at the ASCO meeting, several other pharmaceutical companies have developed KRAS inhibitors that address other cancers that are triggered by KRAS mutations, not only G12C, but also G12D and G13C. Genentech, a division of Roche, has developed a candidate, originally labeled RG330 and renamed divarasib, and reported data from a very small phase 1b study in 29 patients with colorectal cancer. In combination with Lilly’s Erbitux, divarasib led to partial response in 66% of patients, and a combined overall response rate of 62%. Erbitux (cetuximab) is currently the standard of care for colorectal cancer and is also used to treat head and neck cancer. It is an epidermal growth factor (EGFR) inhibitor.
Genentech has said that divarasib’s effectiveness will most likely not be limited to colorectal cancers. They are planning to test it in other forms of cancer where KRAS G12C mutations occur, such as non-small cell lung cancers and other malignancies.
Another KRAS G12C inhibitor as treatment for non-small cell lung cancer and other cancers
This one is from Loxo, an Eli Lilly unit, and is at this point designated LY3537982, so far no name. The new agent was tested alone and in combination with PD-1/L1 inhibitors such as Keytruda. In an 84-patient monotherapy arm, LY3537982 achieved preliminary efficacy at all dose levels and in multiple tumor types, and the efficacy results were similar to those attained by other existing KRAS inhibitors. In eight patients with non-small cell lung cancer who had not previously received a KRAS inhibitor, the disease control rate was 88%. In 14 patients who had received a KRAS inhibitor, the disease control rate was 64%. The Loxo agent also resulted in a 90% disease control rate in the 20 colorectal cancer patients, and a 92% disease control rate in pancreatic cancer. In another category labeled “other” that included ovarian, and head and neck cancers, the disease control rate was 95%.
The term “disease control rate” is somewhat ambiguous. The obvious implication of the term is that the drug controls the disease in that percentage of trial subjects – not necessarily eliminates the disease, but brings it under control. The question is, what defines “under control?” Speaking from my usual skeptical position, I suspect that in cases like this – a new drug up against exceedingly difficult to treat diseases – any significant effect on the severity of symptoms would be classed as disease control.
Keytruda versus Imfinzi for cancers of the bile duct
Cancers of the bile duct – also called “biliary tract cancers” – are rare, but extremely aggressive. The five-year survival rate, as noted earlier, is 17.2%. The two drugs that delivered results that were clinically meaningful, i.e., that the results would have genuine meaning in terms of patients’ lives, were Merck’s Keytruda and AstraZeneca’s Imfinzi.
The results from the Phase 3 KEYNOTE-966 trial demonstrated that adding Keytruda to chemotherapy lowered the risk of death during the trial period by 17% in patients who had previously untreated advanced or metastatic biliary tract cancer. The trial investigators emphasized that the overall survival improvement was statistically significant and clinically meaningful.
Imfinzi did slightly better than Keytruda in the TOPAZ-1 trial, where the risk of death during the trial period was lowered by 20%. Those results gained an FDA blessing in biliary tract cancer in September of 2022.
The Keytruda trial was somewhat larger than the Imfinzi trial, with 1,069 versus 685 randomized patients. However, Imfinzi’s TOPAZ-1 trial had a higher proportion of subjects enrolled in Asia than the KEYNOTE-966 trial, and bile duct cancers are much more prevalent in Asia than in other parts of the world.
In KEYNOTE-966, patients on Keytruda and chemo lived a median 12.7 months, versus 10.9 months for chemo alone. In TOPAZ-1, patients who took Imfinzi alongside chemo enjoyed a median overall survival time of 12.8 months, versus 11.5 months in the control group.
The two-year survival rates were almost identical between the two regimens in their respective trials, both at 25%.
However, the Keytruda regimen’s 14% lower risk of disease progression or death compared with chemo missed the statistical significance bar. Imfinzi, in its own trial, delivered a significant 25% reduction on the same marker.
The gains in median survival time in the KEYNOTE-966 and TOPAZ-1 trials don’t look like a big deal, but they furnish clear evidence that Keytruda and Imfinzi were having a positive effect – reason for further investigation on this usually-fatal cancer.
Tislelizumab versus Keytruda and Opdivo in stomach cancer
Tislelizumab is an anti PD-1/L1 monoclonal antibody co-marketed by Novartis and BeiGene and is being developed globally as a monotherapy and in combination with other therapies for the treatment of a broad array of both solid tumor and hematologic cancers. Keytruda, as you know, comes from Merck and Opdivo from Bristol Myers Squibb.
Preliminary data from the RATIONALE 305 trial showed that tislelizumab and chemotherapy cut the risk of death by 26% compared with chemotherapy alone in patients with PD-1/L1 stomach cancer after a follow-up of about 12 months. Patients on the tislelizumab regimen lived a median 17.2 months versus 12.6 months in the control group. PD-1/L1 is a molecule expressed by some cancer cells to protect the cancer cells against a process termed programmed cell death, which can be initiated by some anti-cancer drugs.
Both Keytruda and Opdivo perform relatively well in stomach cancers. Keytruda plus chemotherapy lowered the risk of death by 22% in gastric of gastroesophageal cancers, regardless of PD-1/L-1 expression, and Opdivo reduced mortality by 20% in those same cancers that were positive for PD-1/L-1. However, in patients with cancers that were negative for PD-/L-1, neither Keytruda nor Opdivo were able to reduce the risk of death by more than 8%.
Tislelizumab’s slight edge (26% versus 20% reduction in the risk of death) over Opdivo in PD-1/L-1-positive stomach cancers may give it a path to FDA approval and a rationale for further research into its potential benefit in other cancers.
News from the prostate cancer front
Prostate cancer is the second-most frequently occurring cancer after breast cancer, with 288,300 estimated cases in 2023. It is also the cancer with the highest five-year survival rate – in the US, 97.4% of men diagnosed with prostate cancer are still alive five years after their diagnosis. This is partly due to the effectiveness of the most common treatment options, which are surgery and radiation. However, the initial opposition of the US Preventive Services Task Force to screening for prostate-specific antigen (PSA), which was rescinded earlier this year, resulted in the progression of prostate cancer in many men to metastatic forms of the cancer. That unfortunate trend in turn led to an increased need for treatment options for advanced and metastatic prostate cancers.
As untreated prostate cancer continues to grow, it can become metastatic, and it can also evade a form of treatment that suppresses the male hormone androgen, which stimulates the growth of the cancer. That form of cancer is termed “castration-resistant prostate cancer” even though patients with castration-resistant prostate cancer are not actually castrated.
Xtandi, a drug from Pfizer and Astellas, is currently used for all stages of castration-resistant prostate cancer and for metastatic hormone-sensitive disease. Recently, data from the Phase 3 EMBARK trial demonstrated the effectiveness of Xtandi in prostate cancer that is not metastatic and that does respond to the male hormone.
Adding Xtandi to the hormone therapy leuprolide reduced the risk of tumor metastasis or death by 58%, compared with leuprolide alone in men with non-metastatic hormone-sensitive prostate cancer. Xtandi alone led to a smaller but still statistically significant reduction of 37% on the same endpoint. Leuprolide is an FDA-approved gonadotropin-releasing hormone agonist used for the management of endometriosis, uterine fibroids, treatment of central precocious puberty in children, and advanced prostate cancer.
Xtandi also demonstrated a trend toward extending lives, although those data weren’t yet mature, meaning that the study had not gone on long enough. The combo and the monotherapy reduced the risk of death by 41% and 23%, respectively, neither of which crossed the statistical significance bar due to the number of subjects enrolled in the trial.
Pfizer is planning to apply for FDA approval of Xtandi for that indication in the coming month.
Meanwhile, the news for AstraZeneca and Merck’s Lynparza in prostate cancer is not so good. FDA reviewers noted that Lynparza only showed a favorable risk-benefit profile in prostate cancer patients with BRCA mutations. For those without BRCA mutations, the reviewers flagged a “modest benefit and possible harm” due to side effects. Most of the benefit in the trial was seen in the 11% of the subjects who had BRCA mutations. In those patients, the Lynparza regimen improved progression-free survival by a whopping 76% and pared down the risk of death by 70%. But in the majority of patients, who were confirmed to be BRCA-negative, progression was staved off in only 15%, which the FDA called “marginal improvement.”
As we’ve said before, pharmaceutical companies are very eager to obtain FDA approval for their candidate drugs, even when the approval is narrow. Approval for a single indication frequently leads to usage in other, related indications – which, in turn can lead to broader approval.
And from the University of California San Diego Health, an improvement in the method of detecting prostate cancer. Instead of the transrectal biopsy, in which the physician puts the biopsy needle through the lining of the rectum to reach the prostate, the needle avoids the rectum and passes through the perineum, which is an area of skin between the base of the scrotum and the rectum. This avoids the risk of introducing fecal material and bacteria into the prostate. Transrectal biopsies carry a 1% to 2% risk of infection, whereas the risk of infection with transperineal biopsies is close to zero. In addition, three-dimensional MRI technology increases the ability to spot potential cancer in the biopsy.
I need to admit that I have a personal interest in prostate cancer detection and treatment. I was diagnosed with prostate cancer about 23 years ago, had the transrectal biopsy with no problems, and (fortunately!) have been entirely cancer-free ever since.
Look for more cancer news in the next installment
Doc Gumshoe has many more news items about current developments in cancer care. His take on those news items will not fit into this installment of the Doc Gumshoe pronouncements. Please look for them in the next issue.
* * * * * * *
To the Gumshoe faithful, I apologize if this opus is over-detailed and replete with medical details of perhaps minor interest. I confess that in the cancer arena I find it difficult to separate the crucial from the incidental. Do please send along whatever comments come into your minds. Be well and keep cool! Best to all, Michael Jorrin (aka Doc Gumshoe)
[ed note: Michael Jorrin, who I dubbed “Doc Gumshoe” many years ago, is a longtime medical writer (not a doctor) and shares his commentary with Gumshoe readers once or twice a month. He does not generally write about the investment prospects of topics he covers, but has agreed to our trading restrictions. Past Doc Gumshoe columns are available here.]